
Independent Prescribing. Pain
Even a child knows how valuable the forest is. The fresh, breathtaking smell of trees. Echoing birds flying above that dense magnitude. A stable climate, a sustainable diverse life and a source of culture. Yet, forests and other ecosystems hang in the balance, threatened to become croplands, pasture, and plantations.
This learning unit is about prescribing and pain.
By completing this learning unity you will be able to:
Describe an overview of the pathophysiology of the nervous system and inflammatory response.
Explain the principles for the transduction of pain
Have an awareness of the differences between nociceptive and neuropathic pain
Consider the principles of pain assessment
Have an awareness of treatment options for pain management including neuropathic pain.
PAIN
Basic types of pain.
Nociceptive,
caused by injury or inflammation.
And is due to the activation of nociceptors. These receptors can respond to heat, cold, vibration, stretch and chemical stimuli released from damaged cells.
The pain signal is transmitted to the brain where it is conceptualised.
Drug treatment aims at blocking the transmission of pain messages to the brain.
Often described as sharpe, aching, throbbing and can be localised, pinpointed.

Neuropathic– nerve related damage or injury of the nerves that transfer information between brain and spinal cord
Originates from pathology of the nervous system. Often described as shooting, burning, toothache and is difficult to localise and can migrate.
Pain
How the body perceives pain
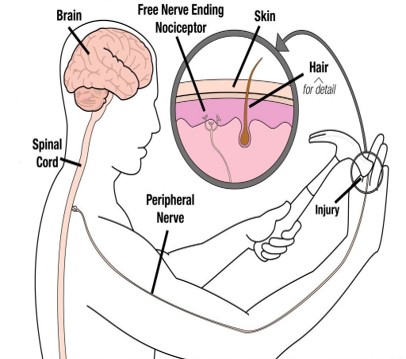
We feel pain through receptors known as Nociceptors.
These Nociceptors are free (bare) nerve endings found in every tissue of the body except the brain ( the skin muscle, joints, bone and viscera). Intense thermal, mechanical or chemical stimuli can activate nociceptors. Nociceptors act as transducers that convert noxious stimuli (pain) into action potentials (have you grasped this concept).
There are several steps in the nociception process:
Transduction- occurs in the periphery – the stimuli
Transmission- an action potential that are transmitted through the nervous system
Modulation – occurs at the dorsal horn of the spinal cord- involves a multitude of neurotransmitters (this bit gets messy).
Perception – final stage resulting in the sensation of pain in the brain.

A dendrite (tree branch) is where a neuron receives input from other cells. Receive synaptic inputs from axons with the input sum determining whether the neuron will fire an action potential
Axon (think root) is the output structure. When a neuron wants to transmit to another neuron it sends an electrical message- action potential through the entire neuron. Action potentials travel down the axon to cause the release of neurotransmitters
The soma (trunk) is where the nucleus lies and DNA housed. Where proteins are made to be transported throughout the axon and dendrites.
There are different types of neurons depending on where they are located, project to and which neurotransmitters they use.
PAIN
ACTION POTENTIAL.
action potential, (here we go): refers to a brief electrical event, typically generated in the axon
Action potential travels the length of the axon to cause the release of neurotransmitters into the synapse
Action potential + neurotransmitter release = communication between neurons.
Voltage gated ion channels
Rapid rise and fall in voltage membrane potential across the cellular membrane- nerve cells have a semi permeable membrane.
The number of steps depends on the text you read. This explanation of action potential for neurons follows three steps. All explanations rely on movement of ions across a membrane.
Na and K both have 1 positive charge +
Ca has 2x positive charges ++
Chloride has 1x negative charge-
Click on the picture for explanation of the three stages

TEST TIME
Lets see what you can remember:
PAIN
Neurotransmission.
Click on the information dots for a basic explanation as to how neurotransmission links to action potential.
More about nociceptive pain
Somatic pain has an external identifiable cause and is transmitted by the Aδ fibres.
activation of nociceptors in skin and skeletal muscle patient can normally identify exactly where the pain is. It can often be reproduced by touching or moving the area or tissue involved.
Visceral pain has an internal cause and is transmitted via the C fibres.
– is activation of the nociceptors in the thoracic or abdominal organs.
-is often poorly localised and may feel like a vague deep ache, sometimes being cramping or colicky in nature.
INFLAMMATION
Initiation of inflationation, in response to soft tissue or muscle injury the body starts a process of chemical signals that initiate responses to aid healing and repair.
The resulting increase in blood flow causes redness (rubor) and increase in heat (calor) at the site of inflammation
The increase permeability of the blood vessels results in exudation of plasma proteins and fluid into the tissue which appears as swelling.

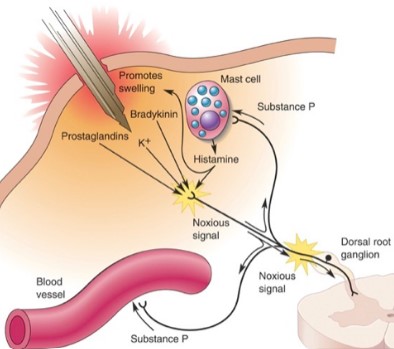
If you cut your hand, several factors contribute to the inflammatory soup
Potassium released from the damaged cells
Prostaglandins, histamines and bradykinin from immune (mast cells) that invade the area during inflammation
Substance P from nearby nerve fibres
Role of Eicosanoids.
Eicosanoids,
are signaling molecules and are composed of a long chain of polyunsaturated fatty acid, eicosapentaenoic acid and dihomo-gamma-linolenic acid but most significantly arachidonic acid.
Eicosanoids play an important role in the body’s inflammatory response, but this is still not clearly understood. What is known is Eicosanoids are involved in vasodilation and vasoconstriction, promotion of sleep, pain and fever.
The Eicosanoids are a large family. Originally named after eico being the ancient Greek number for 20. The ones we are concerned with are:
Leukotrienes
Prostaglandins
Thromboxanes


Prostaglandins are found in most tissues and organs & produced by almost all nucleated cells
Prostaglandins have two derivatives: prostacyclins and thromboxanes.
Prostacyclins are powerful locally acting vasodilators and inhibit the aggregation of blood platelets.
Thromboxane stimulates the formation of a blood clot to try to heal the damage; it also causes the muscle in the blood vessel wall to contract (causing the blood vessel to narrow) to try to prevent blood loss
Prostaglandins are produced following the sequential oxidation of arachidonic acid by cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin synthases.
How Drugs Work
WHO Pain Relief Ladder,
The WHO Pain relief ladder classifies analgesics as
1 Non-opioid
2 Opioid
3 Non-opioid
4 Adjuvant
The same class of drug should not be prescribed at the same time.
How do these different drugs work?

Paracetamol
The exact mechanism of action remains to be determined. There is evidence for a number of central mechanisms. The main ones are:
1.Prostaglandin inhibition: Paracetamol does not have an anti-inflammatory action but it inhibits prostaglandin production. There is a suggestion that unlike NSAIDS paracetamol may act on discrete Cox 1 variant,
2.Cannabinoid: Paracetamol is conjugated to form AM404. This inhibits the reuptake of the endocannabinoid anandamide which increases cannabinoid receptor activation. AM404

NSAIDS
Non-steroidal anti-inflammatory drugs (NSAIDs) reduce the synthesis of prostaglandins, potent sensitizers of primary afferent nociceptors, by inhibiting cyclo-oxygenase which is a key enzyme in the inflammatory cascade. Non-selective COX enzyme inhibitors & can inhibit all types of COX enzymes (COX 1, COX 2 & ?COX 3) E.g. aspirin, ibuprofen, diclofenac, naproxen & available in oral, topical, rectal and IV preparations
The resulting inhibition of prostaglandin and thromboxane synthesis includes:
reduced inflammation
antipyretic
antithrombotic
analgesic effects
The risk of gastrointestinal and other side effects with NSAIDs often prevent their use, particularly in the elderly.

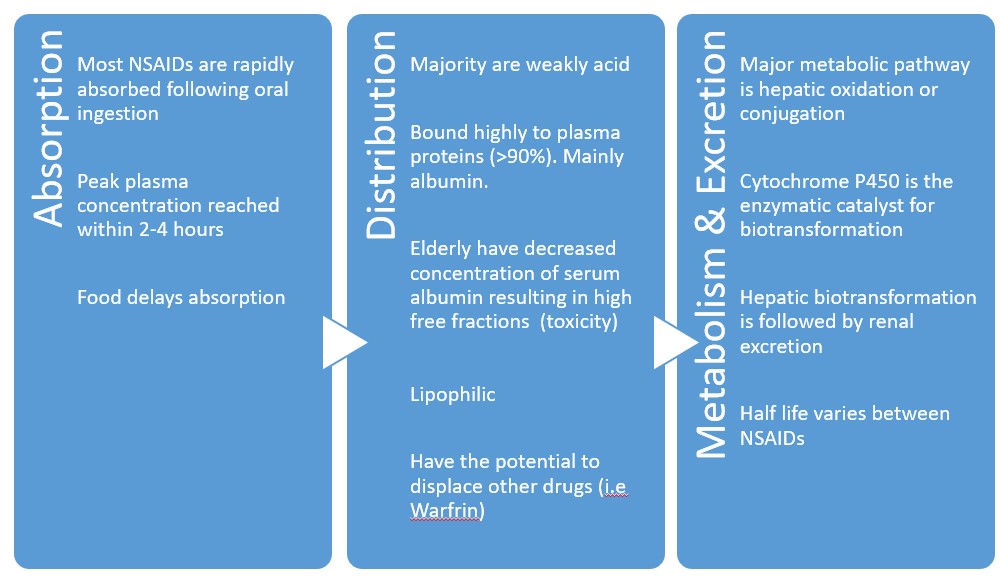
Main adverse effect
Inhibition of COX-1 and COX-2 reduces the levels of protective prostaglandins. This causes increased gastric acid secretion, diminished bicarbonate secretion, diminished mucus secretion.
Common gastrointestinal ADRs include:
– Nausea/vomiting
– Dyspepsia
– Gastric ulceration/bleeding
– Diarrhoea
•Renal
•NSAIDs are associated with a fairly high incidence of renal adverse drug reactions. This is due to changes in renal kidney blood flow, ordinarily mediated by prostaglandins. Resulting in decreased renal perfusion pressure and reduced GFR in pre-existing reduced renal function
Cardiovascular.
Both NSAIDS and selective COX-2 inhibitors are associated with increased risk of myocardial infarction and stroke. Risks increased with increased duration of use. Not recommended in those who have had a previous cardiac event. Rofecoxib (vioxx) withdrawn worldwide October 2004 as showed a statistically significant relative risk of cardiovascular events
ADJUVANTS
Antidepressants: are the mainstay of pharmacological management of neuropathic pain. Tricyclic antidepressants, such as amitriptyline, nortriptyline and imipramine, prevent reuptake of endogenous serotonin and noradrenaline within the central nervous system, increasing the activity of the descending inhibitory pain pathways. Anti-cholinergic side effects predominate, with dry mouth and drowsiness. There is also evidence for the use of duloxetine, a serotonin and noradrenaline reuptake inhibitor (SNRI) in painful diabetic neuropathy.
Anticonvulsants: this group of drugs act either by the blockade of sodium or voltage-gated calcium channels in nerve fibres, reducing excitability of neurons. These drugs may be effective in the management of chronic pain, although frequently cause adverse effects including ataxia, sedation and nausea. Examples include carbamazepine, lamotrigine, gabapentin and pregabalin.
OPIATES
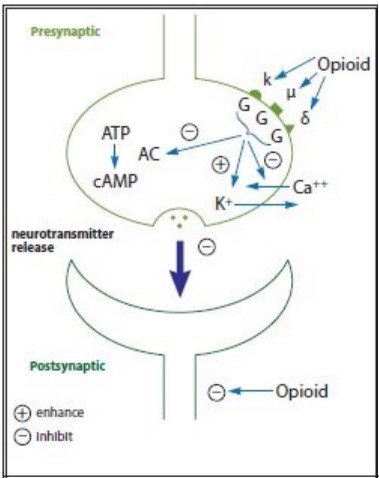
Opioids act as agonist at opioid receptor cites within the CNS and peripheral tissues. These are Mu, Kappa and Delta. Mu receptors are found mainly in the brain stem and are responsible for supraspinal analgesia. Also respiratory depression, euphoria, sedation, reduced gastro motility and physical dependence. Kappa receptors found in limbic areas, brain stem and spinal chord. Responsible for spinal analgesia, sedation, dyspnoea, dysphoria and respiratory depression. Delta receptors are located in the brain and effects are not clearly understood.
Morphine Is a Mu, Kappa and Delta agonist. Activation of the opioid receptors inhibits neural firing and neuro transmitter release. Mu activation inhibits the ascending pain pathway. The Mu excitement is also responsible for the associated euphoric effect of opioids. Metabolites accumulate in renal failure.
Oxycodone is a Mu, Kappa and Delta agonist. Metabolites accumulate in renal failure
Fentanyl is a Mu Kappa and delta agonist, does not have metabolites that accumulate in renal failure.
Tapendtadol is also a Mu receptor agonist and is classed for use in moderate to sever pain. Has comparable effect to oxycodone.
Weak Opiates

ADVERSE EFFECTS: Active opioid metabolites can accumulate in patients who are frail, debilitated or who have significant renal impairment. This can lead to opioid toxicity, characterised by myoclonic jerks, excessive sedation or confusion, restlessness and hallucinations
Respiratory Depression
Constipation
Nausea, vomiting, itching
Drowsiness, mental clouding, dizziness
Hypotensive effect
Abuse, tolerance, dependence & withdrawal
Urinary retention
Codeine is a weak mu and kappa agonist. 100mg of codeine is approximately equivalent to 10 mg or oral morphine.
Tramadol is a weak mu agonist similar to codeine with similar analgesic effect. Also inhibits noradrenaline and serotonin reuptake. It is reported to have less side effects than other mu receptors agonists primarily respiratory depression and gastrointestinal side effects. Tramadol 40mg is approx. 10mg oral morphine. Some texts report 100mg =10mg oral morphine
WHAT TO START PRESCRIBING
WHO Pain Relief Ladder
ASSESSMENT
1 Determine the underlying cause.
2 Evaluate the pain
SOCRATIES


The underlying cause of the pain should be treated whenever possible
A full therapeutic dose should be used before considering switching to a different analgesic, or adding another analgesic.
Caution is needed with long-term use of weak opioids (tolerance and dependence can occur). It may be necessary to reduce the dosage gradually to prevent withdrawal symptoms.
For children (under 16 years of age), either paracetamol or ibuprofen alone are suitable first-line choices
he following treatment options are not recommended for children in primary care:
Administering paracetamol and ibuprofen at the same time.
Naproxen.
Diclofenac.
Aspirin Weak opioids
NON PHARMACOLOGICAL OPTIONS
What are the non- pharmacological options for pain management?
GATE CONTROL THEORY
The dorsal horn of the spinal cord is where primary afferent fibres synapse with 2nd order neurons, complex interactions occur between excitatory and inhibitory interneurons. It is proposed that inhibitory interneurons can be activated by stimulation of non-noxious large sensory afferents from the skin that would then suppress transmission.
Ascending inhibitory pathway
Rubbing- generating lots of touch sensation. These touch signals compete for the synapse- pain message cannot transmit if sensory messages are. One door in a room- lots of people waiting to leave- one person blocking-
One door in a room- lots of people waiting to leave- one person blocking-
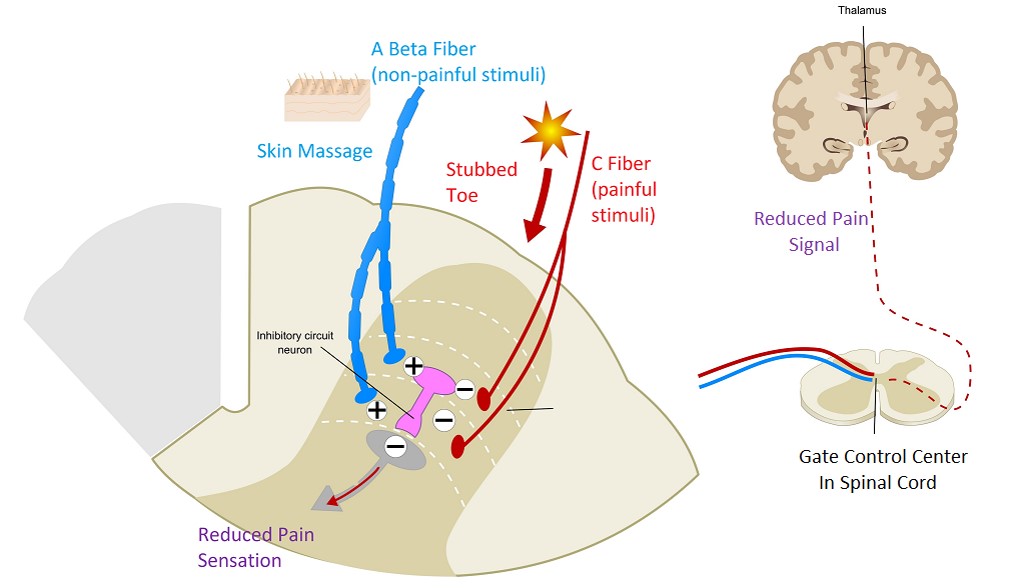
Neuropathic pain management.
Neuropathic pain is very challenging to manage because of the heterogeneity of its aetiologies, symptoms and underlying mechanisms (Beniczky et al. 2005)
●All neuropathic pain (except trigeminal neuralgia)
Offer a choice of amitriptyline, duloxetine, gabapentin or pregabalin as initial treatment for neuropathic pain (except trigeminal neuralgia)
●Trigeminal neuralgia
Offer carbamazepine as initial treatment for trigeminal neuralgia.

Drugs commonly used for Neuropathic Pain:
antidepressants (tricyclic antidepressants [TCAs],
selective serotonin reuptake inhibitors [SSRIs] and
serotonin–norepinephrine reuptake inhibitors [SNRIs]),
antiepileptic (anticonvulsant) drugs,
topical treatments and
opioid analgesics.
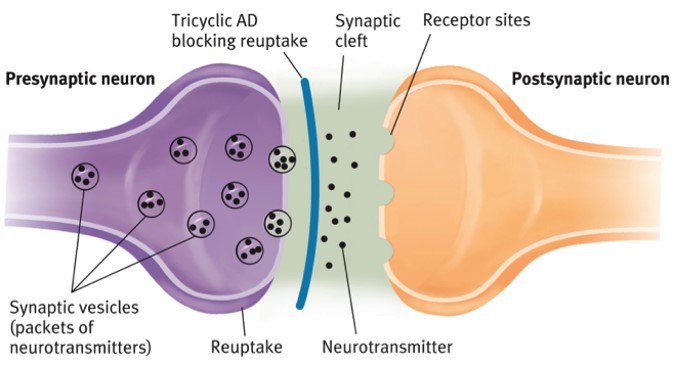
Tricyclic Antidepressants i.e Amitriptyline
The antineuralgic properties of TCAs are independent of their antidepressant properties
Mechanism of action is not entirely understood
Inhibition of Serotonin and noradrenaline reuptake
Know to act on NMDA receptors & Sodium-channel blockade
Excellent bioavailability- once daily administration
Main side effect is sleepiness so should be given at night.
Metabolised by hepatic CYP450 system
GABApentin and Pregabalin
§Simple analogue of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA)
§The elimination half-life is 5-7 hrs
§Mechanism of action for gabapentin is still not fully understood (?voltage gated Ca2+ channels)
§Most trials with pregabalin showed an improvement in neuropathic pain- effect greater with increased doses
§Gabapentin also found to be effective – no dose response identified
Anticonvulsants:
Carbamazepine
§Thought to stabilise inactivated sodium-channels thereby making them less excitable
§Estimated that 90% of patients have a good/excellent initial response and if you are considering this diagnosis and the patient doesn’t respond then you may want to re-think your diagnosis!!
ECOSYSTEM
CHILDREN
§Children’s pain is very different to adults- emotional and psychological factors can affect comprehension and response.
§Assessment is difficult is commonly under-recognised, under-treated and treatment may be delayed in children
§Pain management is under researched so often results in inadequate management
§Insufficient pain relief in newborns/infants cause long term changes in pain perception impacts pain in adulthood.
§Can have long term negative effects- eating/sleeping disorders, increased fears, PTSD


Psychological strategies: involving parents, cuddles, child-friendly environment, and
explanation with reassurance all help build trust.
Distraction with toys, blowing bubbles, reading, or story-telling using superhero or magical imagery to make the pain go away.
§For children (under 16 years of age), either paracetamol or ibuprofen alone are suitable first-line choices
§
The following treatment options are not recommended for children in primary care:
§ Administering paracetamol and ibuprofen at the same time.
§ Naproxen.
§ Diclofenac.
§ Aspirin
§ Weak opioids